The Boiler House

Contents
Water for the Boiler
A steam boiler plant must operate safely, with maximum combustion and heat transfer efficiency. To help achieve this and a long, low-maintenance life, the boiler water can be chemically treated.
Water for the Boiler
The operating objectives for steam boiler plant include:
- Safe operation.
- Maximum combustion and heat transfer efficiency.
- Minimum maintenance.
- Long working life.
The quality of the water used to produce the steam in the boiler will have a profound effect on meeting these objectives.
There is a need for the boiler to operate under the following criteria:
- Freedom from scale - If hardness is present in the feedwater and not controlled chemically, then scaling of the heat transfer surfaces will occur, reducing heat transfer and efficiency - making frequent cleaning of the boiler necessary. In extreme cases, local hot spots can occur, leading to mechanical damage or even tube failure.
- Freedom from corrosion and chemical attack - If the water contains dissolved gases, particularly oxygen, corrosion of the boiler surfaces, piping and other equipment is likely to occur.
If the pH value of the water is too low, the acidic solution will attack metal surfaces. If the pH value is too high, and the water is alkaline, other problems such as foaming may occur.
Caustic embrittlement or caustic cracking must also be prevented in order to avoid metal failure. Cracking and embrittlement are caused by too high a concentration of sodium hydroxide. Older riveted boilers are more susceptible to this kind of attack; however, care is still necessary on modern welded boilers at the tube ends.kind of attack; however, care is still necessary on modern welded boilers at the tube ends.
Good quality steam
If the impurities in the boiler feedwater are not dealt with properly, carryover of boiler water into the steam system can occur. This may lead to problems elsewhere in the steam system, such as:
- Contamination of the surfaces of control valves - This will affect their operation and reduce their capacity.
- Contamination of the heat transfer surfaces of process plant - This will increase thermal resistance, and reduce the effectiveness of heat transfer.
- Restriction of steam trap orifices - This will reduce steam trap capacities, and ultimately lead to waterlogging of the plant, and reduced output.
Carryover can be caused by two factors:
1. Priming - This is the ejection of boiler water into the steam take-off and is generally due to one or more of the following:
-Operating the boiler with too high a water level.
-Operating the boiler below its design pressure; this increases the volume and the velocity of the steam released from the water surface.
-Excessive steam demand.
2. Foaming - This is the formation of foam in the space between the water surface and the steam off-take. The greater the amount of foaming, the greater the problems which will be experienced.
The following are indications and consequences of foaming:
-Water will trickle down from the steam connection of the gauge glass; this makes it difficult to accurately determine the water level.
-Level probes, floats and differential pressure cells have difficulty in accurately determining water level.
-Alarms may be sounded, and the burner(s) may even ‘lockout’. This will require manual resetting of the boiler control panel before supply can be re-established.
These problems may be completely or in part due to foaming in the boiler. However, because foaming is endemic to boiler water, a better understanding of foam itself is required:
- Surface definition - Foam on a glass of beer sits on top of the liquid, and the liquid/foam interface is clearly defined. In a boiling liquid, the liquid surface is indistinct, varying from a few small steam bubbles at the bottom of the vessel, to many large steam bubbles at the top.
- Agitation increases foaming - The trend is towards smaller boilers for a given steaming rate. Smaller boilers have less water surface area, so the rate at which steam is released per square metre of water area is increased. This means that the agitation at the surface is greater. It follows then that smaller boilers are more prone to foaming.
- Hardness - Hard water does not foam. However, boiler water is deliberately softened to prevent scale formation, and this gives it a propensity to foam.
- Colloidal substances - Contamination of boiler water with a colloid in suspension, for example. milk, causes violent foaming. Note: Colloidal particles are less than 0.000 1 mm in diameter, and can pass through a normal filter.
- TDS level - As the boiler water TDS increases, the steam bubbles become more stable, and are more reluctant to burst and separate.
Corrective action against carryover
The following alternatives are open to the Engineering Manager to minimise foaming in the boiler:
- Operation - Smooth boiler operation is important. With a boiler operating under constant load and within its design parameters, the amount of entrained moisture carried over with steam may be less than 2%.
If load changes are rapid and of large magnitude, the pressure in the boiler can drop considerably, initiating extremely turbulent conditions as the contents of the boiler flash to steam. To make matters worse, the reduction in pressure also means that the specific volume of the steam is increased, and the foam bubbles are proportionally larger.
If the plant conditions are such that substantial changes in load are normal, it may be prudent to consider:
-Modulating boiler water level controls if on/off are currently fitted.
-‘Surplussing controls’ that will limit the level to which the boiler pressure is allowed to drop.
-A steam accumulator (see Module 22 of this Block).
-‘Feed-forward’ controls that will bring the boiler up to maximum operating pressure before the load is applied.
-‘Slow-opening’ controls that will bring plant on-line over a pre-determined period.
- Chemical control - Anti-foaming agents may be added to the boiler water. These operate by breaking down the foam bubbles. However, these agents are not effective when treating foams caused by suspended solids.
- Control of TDS - A balance has to be found between:
-A high TDS level with its attendant economy of operation.
-A low TDS level which minimises foaming.
- Safety - The dangers of overheating due to scale, and of corrosion due to dissolved gases, are easy to understand. In extreme cases, foaming, scale and sludge formation can lead to the boiler water level controls sensing improper levels, creating a danger to personnel and process alike.
External water treatment
It is generally agreed that where possible on steam boilers, the principal feedwater treatment should be external to the boiler.
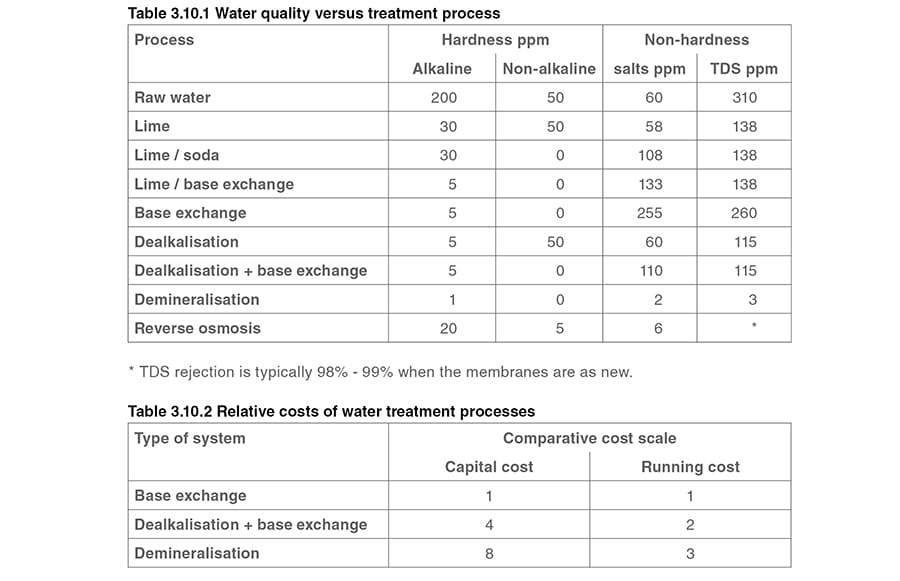
A summary of the treated water quality that might be obtained from the various processes, based on a typical hard raw water supply, is shown in Table 3.9.2. This is the water that the external treatment plant has to deal with.
External water treatment processes can be listed as:
- Reverse osmosis - A process where pure water is forced through a semi-permeable membrane leaving a concentrated solution of impurities, which is rejected to waste.
- Lime; lime/soda softening - With lime softening, hydrated lime (calcium hydroxide) reacts with calcium and magnesium bicarbonates to form a removable sludge. This reduces the alkaline (temporary) hardness. Lime/soda (soda ash) softening reduces non-alkaline (permanent) hardness by chemical reaction.
- Ion exchange - Is by far the most widely used method of water treatment for shell boilers producing saturated steam. This module will concentrate on the following processes by which water is treated: Base exchange, Dealkalisation and Demineralisation.
Ion exchange
An ion exchanger is an insoluble material normally made in the form of resin beads of 0.5 to 1.0 mm diameter. The resin beads are usually employed in the form of a packed bed contained in a glass reinforced plastic pressure vessel. The resin beads are porous and hydrophilic - that is, they absorb water. Within the bead structure are fixed ionic groups with which are associated mobile exchangeable ions of opposite charge. These mobile ions can be replaced by similarly charged ions, from the salts dissolved in the water surrounding the beads.
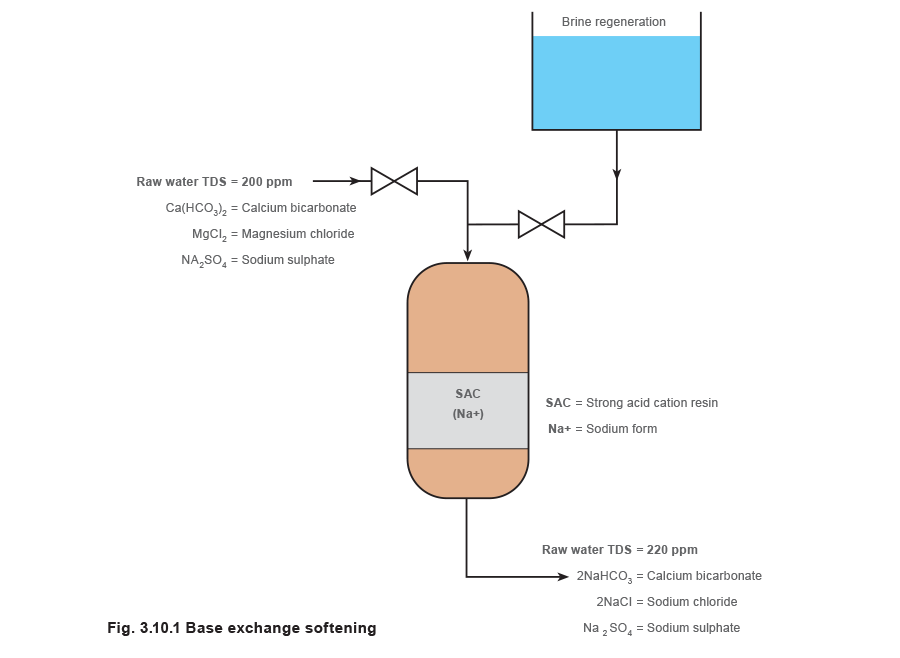
Base exchange softening
This is the simplest form of ion exchange and also the most widely used. The resin bed is initially activated (charged) by passing a 7 - 12% solution of brine (sodium chloride or common salt) through it, which leaves the resin rich in sodium ions. Thereafter, the water to be softened is pumped through the resin bed and ion exchange occurs. Calcium and magnesium ions displace sodium ions from the resin, leaving the flowing water rich in sodium salts. Sodium salts stay in solution at very high concentrations and temperatures and do not form harmful scale in the boiler.
From Figure 3.10.1 it can be seen that the total hardness ions are exchanged for sodium. With sodium base exchange softening there is no reduction in the total dissolved solids level (TDS in parts per million or ppm) and no change in the pH. All that has happened is an exchange of one group of potentially harmful scale forming salts for another type of less harmful, non-scale forming salts. As there is no change in the TDS level, resin bed exhaustion cannot be detected by a rise in conductivity (TDS and conductivity are related). Regeneration is therefore activated on a time or total flow basis.
Softeners are relatively cheap to operate and can produce treated water reliably for many years. They can be used successfully even in high alkaline (temporary) hardness areas provided that at least 50% of condensate is returned. Where there is little or no condensate return, a more sophisticated type of ion exchange is preferable.
Sometimes a lime/soda softening treatment is employed as a pre-treatment before base exchange. This reduces the load on the resins.
Dealkalisation
The disadvantage of base exchange softening is that there is no reduction in the TDS and alkalinity. This may be overcome by the prior removal of the alkalinity and this is usually achieved through the use of a dealkaliser.
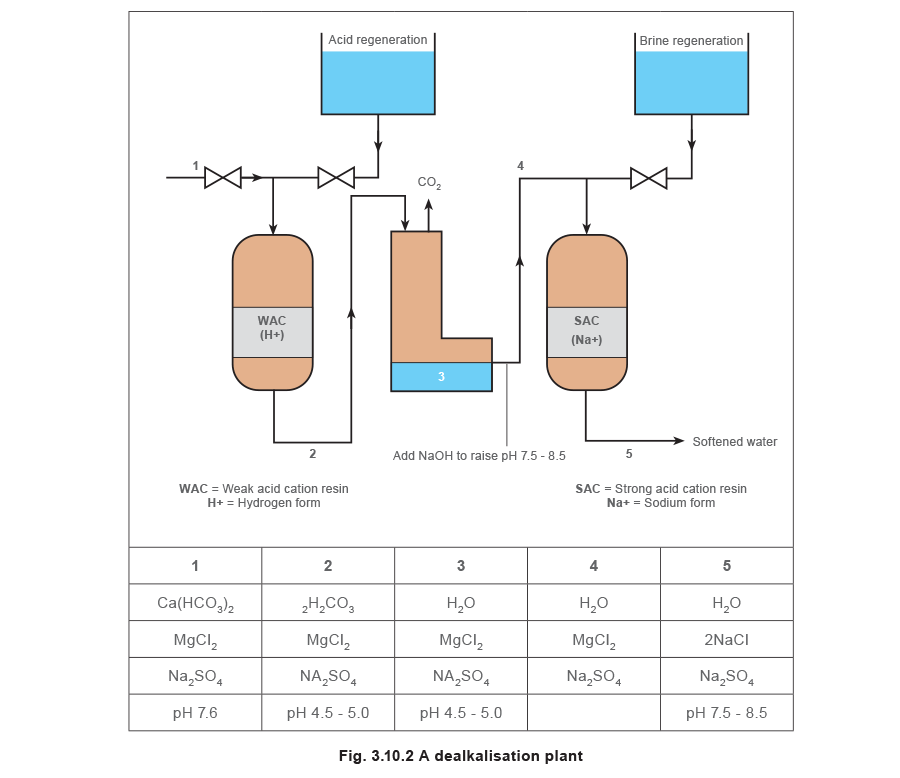
There are several types of dealkaliser but the most common variety is shown in Figure 3.10.2. It is really a set of three units, a dealkaliser, followed by a degasser and then a base exchange softener.
Dealkaliser
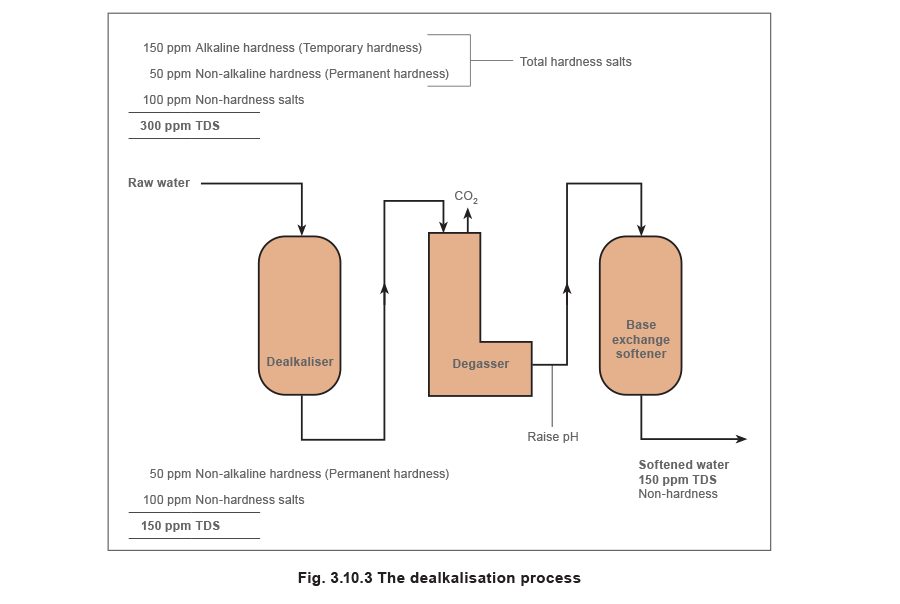
The system shown in Figure 3.10.3 is sometimes called ‘split-stream’ softening. A dealkaliser would seldom be used without a base exchange softener, as the solution produced is acidic and would cause corrosion, and any permanent hardness would pass straight into the boiler.
A dealkalisation plant will remove temporary hardness as shown in Figure 3.10.3. This system would generally be employed when a very high percentage of make-up water is to be used.
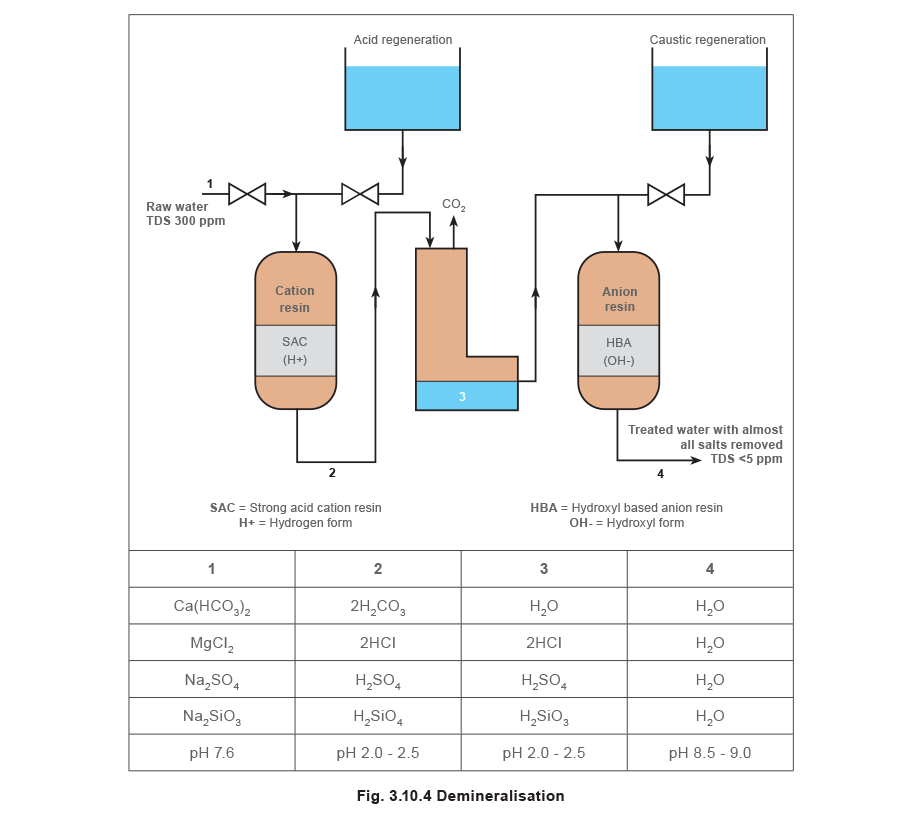
Demineralisation
This process will remove virtually all the salts. It involves passing the raw water through both cation and anion exchange resins (Figure 3.10.4). Sometimes the resins may be contained in one vessel and this is termed ‘mixed bed’ demineralisation.
The process removes virtually all the minerals and produces very high quality water containing almost no dissolved solids. It is used for very high pressure boilers such as those in power stations.
If the raw water has a high amount of suspended solids this will quickly foul the ion exchange material, drastically increasing operating costs. In these cases, some pre-treatment of the raw water such as clarification or filtration may be necessary.
Selection of external water treatment plant
Looking at Table 3.10.1, it is tempting to think that a demineralisation plant should always be used. However, each system has a capital cost and a running cost, as Table 3.10.2 illustrates, plus the demands of the individual plant need to be evaluated.
Shell boiler plant
Generally, shell boilers are able to tolerate a fairly high TDS level, and the relatively low capital and running costs of base-exchange softening plants (see Table 3.10.2) will usually make them the first choice.
If the raw water supply has a high TDS value, and/or the condensate return rate is low (<40%), there are a few options which may be considered:
- Pre-treatment with lime/soda which will cause the alkaline hardness to precipitate out of solution as calcium carbonate and magnesium hydroxide, and then drain from the reaction vessel.
- A dealkalisation plant to reduce the TDS level of the water supplied to the boiler plant.
Water-tube boiler plant
Water-tube boiler plant is much less tolerant of high TDS levels, and even less so as the pressure increases. This is due to a number of reasons, including:
- Water-tube boilers have a limited water surface area in the steam drum, relative to the evaporation rate.
This results in very high steam release rates per unit of water area, and turbulence.
- Water-tube boilers tend to be higher rated, perhaps over 1 000 tonnes/h of steam. This means that even a small percentage blowdown can represent a high mass to be blown down.
- Water-tube boilers tend to operate at higher pressures, usually up to 150 bar g. The higher the pressure, the greater the energy contained in the blowdown water.
Higher pressures also mean higher temperatures. This means that the materials of construction will be subjected to higher thermal stresses, and be operating closer to their metallurgical limitations. Even a small amount of internal contamination hindering the heat transfer from tubes to water may result in the tubes overheating.
- Water-tube boilers often incorporate a superheater.
The dry saturated steam from the steam drum may be directed to a superheater tubes situated in the highest temperature area of the furnace. Any carryover of contaminated water with the steam would coat the inside of the superheater tubes, and inhibit heat transfer with potentially disastrous results.
The above factors mean that:
- High quality water treatment is essential for the safe operation of this type of plant.
- It may be economically viable to invest in a water treatment plant that will minimise blowdown rates.
In each of these cases, the selection will often be a demineralisation or a reverse osmosis plant.
Summary
The quality of raw water is obviously an important factor when choosing a water treatment plant. Although TDS levels will affect the performance of the boiler operation, other issues, such as total alkalinity or silica content can sometimes be more important and then dominate the selection process for water treatment equipment.